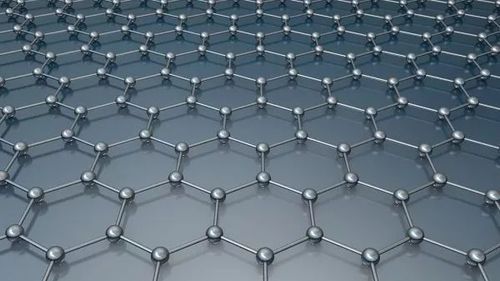
Graphene is a two-dimensional material that has revolutionized the field of materials science due to its unique properties, such as high strength, excellent electrical conductivity, and lightweight nature. Carbon, on the other hand, is a carbon-based molecule that has been used for centuries in various applications, including fuel production, construction, and electronics.
(is graphene similar to carbon)
While both Graphene and Carbon share many similarities, there are also significant differences between them. One of the most notable differences is their structure. Graphene is an atomic crystal structure, meaning that each atom is surrounded by six other atoms and arranged in a hexagonal lattice. This arrangement gives graphene its unique properties, such as high strength and flexibility.
Carbon, on the other hand, is a linear molecule made up of carbon atoms. The carbon atoms are bonded together to form a three-dimensional network structure. This arrangement allows Carbon to be used in a wide range of applications, including fuels, plastics, and textiles.
Another similarity between Graphene and Carbon is their chemical behavior. Both Graphene and Carbon are highly reactive materials, capable of forming strong bonds with other molecules. However, the specific type of bond formed by Graphene is different from the type of bond formed by Carbon.
In terms of physical properties, Graphene is generally considered to be stronger than Carbon. A study published in Nature Materials found that Graphene was able to resist more mechanical stress than Carbon under similar conditions. Additionally, Graphene has a lower surface area to volume ratio compared to Carbon, which means it can carry more electrical charge per unit area.
Despite these similarities, there are still some important differences between Graphene and Carbon. For example, Graphene has a higher melting point than Carbon, indicating that it requires more energy to change from a solid to a liquid state. Additionally, Graphene has a lower thermal conductivity than Carbon, suggesting that it is less efficient at dissipating heat.
(is graphene similar to carbon)
Overall, while there are similarities between Graphene and Carbon, there are also significant differences. While both materials have unique properties, they are different in their structure, chemistry, and physical properties. Understanding these differences will help scientists develop new materials and technologies that leverage the strengths of both Graphene and Carbon.
Inquiry us