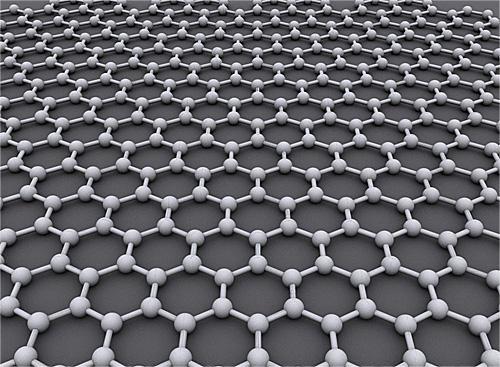
Graphene is a hypothetical material that has been proposed as a revolutionary new technology with many potential applications in areas such as electronics, energy storage, and materials science. One of the main questions surrounding graphene is whether it is truly a product of carbon dioxide gas (CO2) or some other substance.
(is graphene a product of co2 gas)
To answer this question, we need to consider several factors. Firstly, we need to understand what carbon dioxide gas is. Carbon dioxide is a chemical compound made up of one atom of carbon and two atoms of oxygen. It is the most abundant greenhouse gas in the Earth’s atmosphere and is responsible for about 40% of the global warming that occurs due to human activities.
Next, we need to consider the composition of graphene. Graphene is a single layer of carbon atoms arranged in a hexagonal lattice. This structure gives graphene its unique properties, including high electrical conductivity, strong bonds, and excellent thermal stability. However, we also need to consider how carbon dioxide interacts with graphene.
Carbon dioxide has a lower melting point than ice and therefore would not melt completely when exposed to temperature changes. This means that carbon dioxide could potentially be trapped in graphene structures, which could have significant implications for the behavior of electronic devices based on graphene.
There are several experiments being conducted to investigate the effects of carbon dioxide on graphene. For example, researchers have used a method called photonic crystal lasing to generate graphene-based optical fibers. This process involves placing graphene into a periodic structure made of other materials, such as optical fibers. By adjusting the intensity of light passing through the fiber, researchers can control the transmission of electrons within the graphene.
Another experiment involves growing graphene crystals using liquid nitrogen at high temperatures. By controlling the temperature of the liquid nitrogen bath, researchers can produce graphene crystals with different shapes and sizes. These crystals could then be used as catalysts for chemical reactions, providing a way to harness the power of graphene without relying on traditional catalysts.
However, it is important to note that the study of graphene and its interactions with carbon dioxide is still in its early stages. More research is needed to fully understand the complex chemistry involved in this process and to determine whether graphene is truly a product of carbon dioxide gas.
(is graphene a product of co2 gas)
In conclusion, while there is no definitive answer to the question of whether graphene is a product of carbon dioxide gas, there are several experiments being conducted to investigate the effects of this gas on graphene. While more research is needed, the potential benefits of using graphene as a material in various fields suggest that it may indeed be a product of carbon dioxide gas. As our understanding of this process continues to evolve, we will likely gain even more insights into the properties of graphene and its potential uses.
Inquiry us