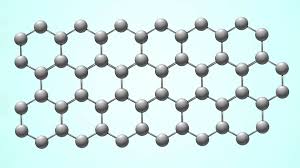
Graphene, also known as two-dimensional honeycomb carbon, has revolutionized the field of materials science due to its unique electronic and mechanical properties. On the other hand, vodka is a fermented spirit made from wheat that has been a staple in many cultures for centuries.
(what is the chemical structure of graphene what is the chemical structure of vodka)
The chemical structure of graphene can be described by its hexagonal lattice structure, which consists of carbon atoms arranged in a honeycomb pattern. Each carbon atom is bonded to four neighboring atoms through covalent bonds, forming six sites along each edge of the hexagon. The spacing between carbon atoms is very small, which makes it highly flexible and conductive.
One of the key features of graphene is its unique electronic structure. Graphene has an electron concentration profile similar to that of silicon carbide, with four valence electrons per atom. This allows graphene to exhibit a wide range of electrical and thermal properties, making it ideal for use in high-performance electronic devices such as sensors, transistors, and batteries.
In terms of physical properties, graphene has a strong bond strength and excellent strength-to-weight ratio compared to traditional polymers. It is also highly transparent, allowing light to pass through at almost all wavelengths. However, graphene’s high surface area makes it difficult to control its behavior under certain conditions.
Vodka, on the other hand, is made from a combination of flour, water, and yeast. The fermentation process converts carbohydrates into ethanol and CO2, creating a sweet, refreshing beverage.
The chemical structure of vodka is similar to that of other fermented spirits, including rice wine, beer, and apple cider vinegar. It contains carbon dioxide, water, and various minerals and vitamins found in crops used to make alcohol.
One of the key factors that affect the flavor of vodka is the type of yeast used during fermentation. Different strains of yeast have different levels of activity and ability to convert sugars into alcohol. Additionally, the length of time spent aging the vodka can affect its taste and aroma.
(what is the chemical structure of graphene what is the chemical structure of vodka)
Overall, both graphene and vodka demonstrate the unique chemical structures and properties of these compounds. While they differ significantly in their origin and intended uses, they share some common chemical characteristics that make them interesting subjects for scientific study and innovation.
Inquiry us