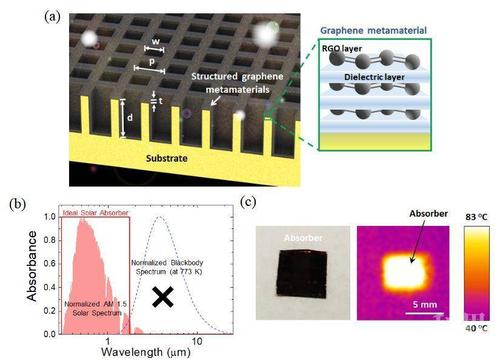
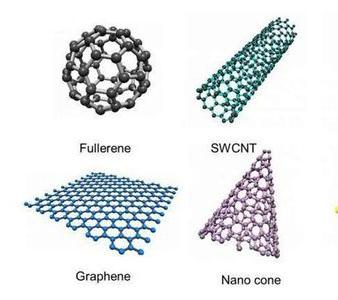
Graphene is a two-dimensional material that has attracted significant attention due to its unique properties and potential applications. Graphene is made up of carbon atoms arranged in a hexagonal lattice structure, which gives it strong adsorption abilities for various molecules and is also incredibly lightweight.
(what elements make up graphene)
One of the key elements that makes up graphene is carbon. Carbon is the building block of all organic matter, including proteins, nucleic acids, and carbohydrates. It forms a vast array of covalent bonds with other atoms, making it possible for carbon atoms to form strong interatomic connections. This strength of the carbon bond allows graphene to be highly flexible and responsive to external stimuli.
Another important element in graphene is the hydrogen atom, which acts as a covalent bonding center between the carbon atoms. Hydrogen bonds are extremely weak, but they can still hold together the individual atoms in a graphene sheet. The hydrogen atoms also provide an additional layer of stability and resistance to water and other chemicals.
Graphene is composed of layers of carbon atoms, each consisting of several hundred to several thousand carbon atoms. These layers are stacked together to form a single layer, and each layer is separated by air or a layer of another material such as a polymer. The number of layers in a graphene sheet depends on its thickness and composition, with smaller sheets typically having more layers than larger ones.
In addition to these core elements, graphene is also rich in functional groups such as oxygen, nitrogen, sulfur, and phosphorus. These functional groups provide a range of unique properties that make graphene suitable for a variety of applications.
One of the most promising applications of graphene is in electronics. Graphene has a very low electrical conductivity, which means that it can be used to create highly conductive electronic components such as transistors, sensors, and memory devices. Additionally, graphene’s high surface area provides excellent electric field distribution and conductance, making it ideal for use in superconducting circuits and hybrid electronics.
Graphene is also being studied for its potential use in energy storage. Graphene has a high specific heat capacity, which means that it can store large amounts of energy per unit volume. This could make graphene a promising material for use in batteries and fuel cells.
Another application of graphene is in medicine. Graphene has the potential to revolutionize the way we treat diseases by providing new avenues for drug delivery and improving diagnosis and treatment methods. For example, graphene-based materials have been proposed as a way to deliver drugs directly to cancer cells, while graphene-based sensors can be used to detect disease biomarkers at the molecular level.
(what elements make up graphene)
Despite its many potential applications, graphene is still in the early stages of development. Researchers are working to improve the material’s properties, reduce its cost, and overcome its limited availability. However, with continued research and development, graphene is likely to play a major role in many fields in the coming years.
Inquiry us