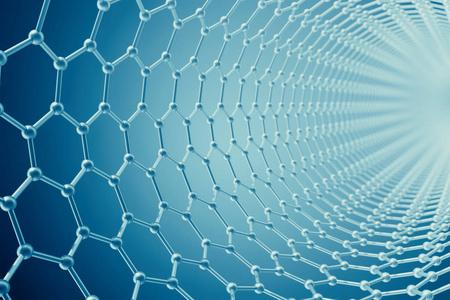
Graphene is a single layer of carbon atoms arranged in a hexagonal lattice, which gives it unique properties such as strength, flexibility, and conductivity. It has attracted interest from scientists for its potential applications in fields such as electronics, energy storage, and nanotechnology.
(can an atom of hydrogen pass through graphene)
However, the question remains: can an atom of hydrogen pass through graphene? While hydrogen atoms are not the same as carbon atoms in terms of size and shape, they do share some similarities with them. For example, both hydrogen and carbon have a shell structure, which consists of two electrons orbiting around the nucleus in a closed loop. Additionally, both hydrogen and carbon have bonding pairs that form covalent bonds between their atoms.
One way to think about this is to consider how hydrogen interacts with graphene. Hydrogen is a nonpolar molecule, so it does not interact strongly with polar molecules like carbon. However, there are still ways in which hydrogen could interact with carbon, depending on the specific conditions.
One possibility is that hydrogen can act as a catalyst for chemical reactions within graphene. Carbon is known to be susceptible to electron transfer reactions, where an atom loses or gains electrons to form a new compound. If hydrogen were added to a graphene-based system, it could potentially accelerate these reactions by forming strong electrostatic bonds with carbon atoms.
Another possibility is that hydrogen could act as a dielectric material, which could influence the electrical conductivity of graphene. Graphene is known to be an insulator, meaning that it does not allow electric current to flow easily. However, if hydrogen were added to a graphene-based system, it could potentially increase its resistance to electric current flow, making it more suitable for use as an electronic conductor.
(can an atom of hydrogen pass through graphene)
Overall, while an atom of hydrogen cannot directly pass through graphene, it could still interact with the molecule in some limited ways. By understanding the interactions between hydrogen and carbon at the atomic level, we may be able to develop new technologies and applications that take advantage of the unique properties of graphene.
Inquiry us