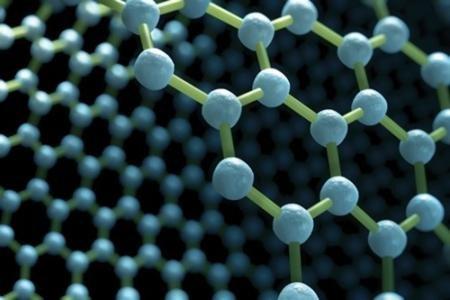
Graphene is a two-dimensional material that has gained significant attention in recent years due to its unique properties. One of these properties is its excellent thermal conductivity, which makes it an ideal material for heat transfer applications such as cooling systems and electronics.
(What bond of graphene makes it have good thermal properties)
One of the key factors that contribute to the high thermal conductivity of graphene is its structure. Graphene consists of layers of carbon atoms arranged in a hexagonal lattice, which creates a low surface area. This lower surface area allows more electrons to be packed into each unit cell, resulting in a higher thermal conductivity.
Another factor that contributes to the high thermal conductivity of graphene is its very small size. The thickness of a single layer of graphene is on the order of nanometers, meaning that it is extremely thin. This thinness results in a large number of free electrons, which can carry heat easily through the material.
In addition to its excellent thermal conductivity, graphene also exhibits exceptional electrical conductivity. This means that it can conduct electricity with high speed and without resistance, making it an ideal material for use in electronic devices such as sensors and transistors.
(What bond of graphene makes it have good thermal properties)
Overall, the bond of graphene’s high thermal conductivity is due to its unique structure and very small size. These properties make graphene an ideal material for a wide range of thermal and electrical applications. As researchers continue to explore the properties of graphene, we can expect to see even more innovative uses for this fascinating material.
Inquiry us