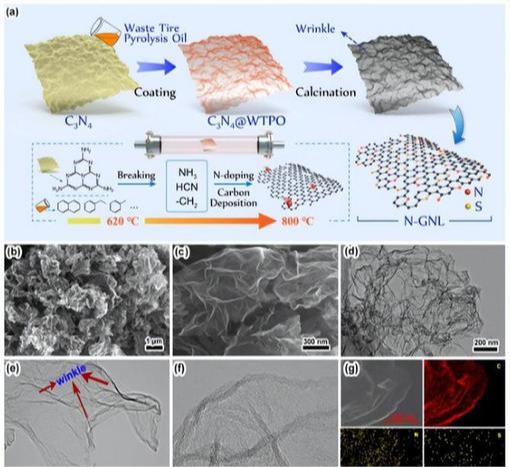
Graphene, the unique and powerful material that has captured the attention of scientists and engineers for its incredible properties, is actually an allotrope of carbon. While other forms of carbon, such as diamond or bamboo, are often considered to be precursors to graphene, this distinction is important to understand.
(what is not an allotrope of carbon is graphene an allotrope of carbon)
An allotrope refers to a type of crystal structure in which the atoms or molecules in a substance have been arranged in a repeating pattern, similar to how atoms in a regular arrangement form a molecule in a crystal. This means that the same arrangement of atoms can exist in different types of materials, but each type will have its own distinct physical properties.
For example, the carbon atoms in diamond are arranged in a regular pattern known as the octahedral lattice, while the carbon atoms in bamboo are arranged in a hexagonal lattice. These two types of materials are significantly different in their physical properties, such as hardness, strength, and flexibility, due to the difference in the arrangement of atoms.
However, graphene is not an allotrope of carbon. Graphene is a single layer of carbon atoms arranged in a honeycomb-like structure, with each atom bonded to three neighboring atoms through covalent bonds. This unique structure allows graphene to exhibit exceptional electrical and mechanical properties, making it a promising material for a wide range of applications.
In contrast, diamonds and bamboo do not have this unique structure. While diamonds and bamboo can be made using traditional chemical methods, they are not considered to be natural occurring substances. Instead, they are man-made materials created by industrial processes.
It’s worth noting that the term “allotrope” can also refer to a particular subset of a larger set of possible structures or arrangements. For example, some scientists consider carbon to be a type of amorphous material, meaning that it does not have a well-defined crystal structure like diamond or bamboo. Other scientists might use the term “allotrope” more broadly to describe all carbon structures that share some similarities with regular carbon crystals.
(what is not an allotrope of carbon is graphene an allotrope of carbon)
In conclusion, while grapheme is an important and versatile material with a unique crystal structure, it is not an allotrope of carbon. Instead, it is a single layer of carbon atoms arranged in a honeycomb-like structure, with exceptional electrical and mechanical properties. Understanding the differences between these materials can help us better appreciate their unique characteristics and potential applications.
Inquiry us