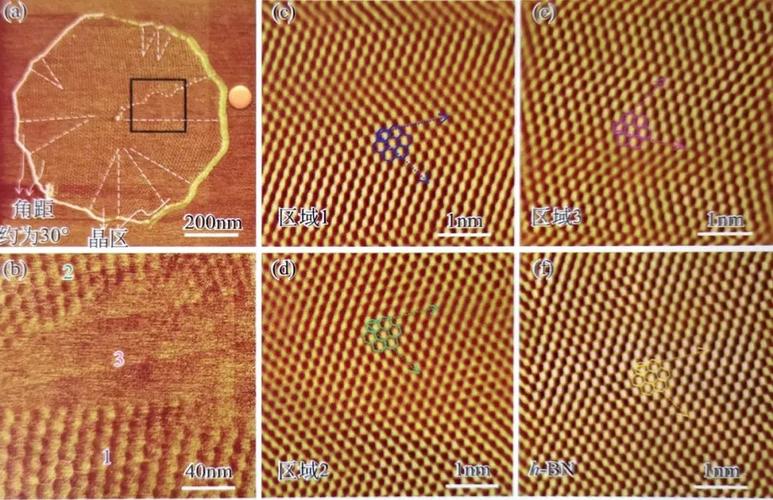
Graphene is a two-dimensional material that has attracted significant attention due to its unique properties, particularly in the field of materials science and engineering. Graphene is composed of carbon atoms arranged in a hexagonal lattice, which allows it to possess extraordinary mechanical strength and electrical conductivity.
(what makes graphene so strong)
One of the most remarkable features of graphene is its exceptional mechanical strength. The carbon atoms in a graphene sheet are arranged in such a way that they interlock in a highly ordered manner, which gives rise to a strong bond between adjacent layers. This results in a high Young’s modulus (the measure of the rigidity of a material) and excellent tensile strength, making it capable of great forces without breaking.
Another important property of graphene is its high electrical conductivity. Graphene has an electric conductivity that is on par with that of copper, making it an ideal material for use in electronic devices such as transistors and sensors. This property also makes graphene useful for creating high-speed circuits and enabling the development of new types of electronics.
Despite its impressive mechanical and electrical properties, graphene has several other unique features that make it an attractive material for various applications. For example, graphene can be used as a catalyst for chemical reactions, as well as for fuel cells and solar panels. It is also biocompatible, meaning that it does not harm living organisms when introduced into their environment.
(what makes graphene so strong)
In conclusion, graphene is a highly promising material that offers a wide range of potential applications in fields such as materials science, engineering, and medicine. Its exceptional mechanical and electrical properties, along with its unique, make it an exciting material that is likely to have a significant impact on the future of technology.