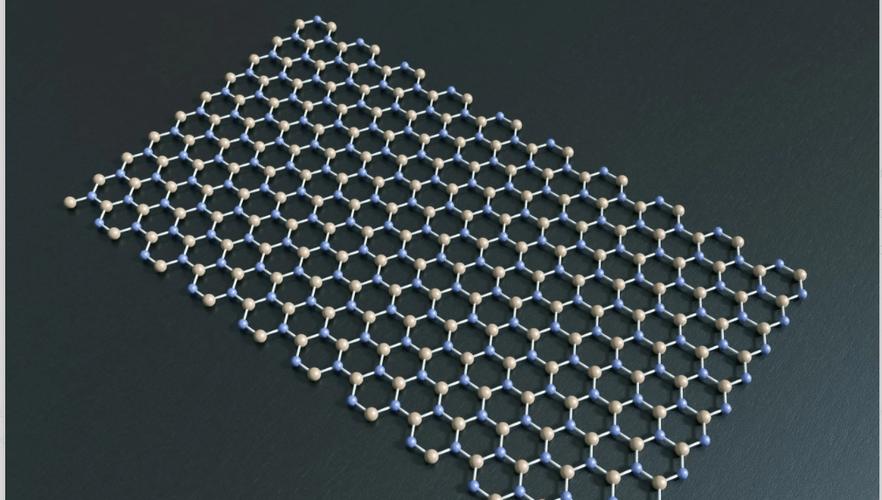
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, has been attracting researchers and scientists for its unique properties. One of the most interesting things about graphene is its exceptional electrical conductivity, which can be up to several times higher than that of copper or silver. This high conductivity is due to the unique structure of the carbon atoms in the graphene sheet, which allows them to form strong electronic bonds.
(what type of bond is in graphene)
There are several types of bonds that can be formed between carbon atoms in graphene, but one of the most important is the covalent bond. In this type of bond, each carbon atom in the graphene sheet forms a stable bond with every other carbon atom in the sheet. This means that graphene has a highly efficient energy transfer mechanism, making it well-suited for use in electronic devices.
Another type of bond that can be formed between carbon atoms in graphene is an ionic bond. In an ionic bond, one carbon atom is replaced by an ionized atom (such as an electron) and the other carbon atom is replaced by a neutral atom. The electric charge on the ionized atom attracts the charges on the neutral atoms, forming a strong electrostatic bond. Ionic bonds have some advantages over covalent bonds, such as being chemically stable and having a high strength.
The last type of bond that can be formed between carbon atoms in graphene is a hydrogen bond. In a hydrogen bond, two carbon atoms form a strong bond with a bond angle of around 147 degrees. Hydrogen bonds are relatively weak compared to other types of bonds, but they are still useful in certain situations. For example, hydrogen bonds can be used to stabilize small molecules and colloids.
(what type of bond is in graphene)
Overall, the type of bond that can be formed between carbon atoms in graphene depends on the specific arrangement of the carbon atoms in the sheet. There are several different types of bonds that can be formed, including covalent bonds, ionic bonds, and hydrogen bonds. Each type of bond has its own unique properties and is well-suited for different applications. As research into graphene continues, we will likely learn more about the different types of bonds that can be formed between carbon atoms in this remarkable material.
Inquiry us