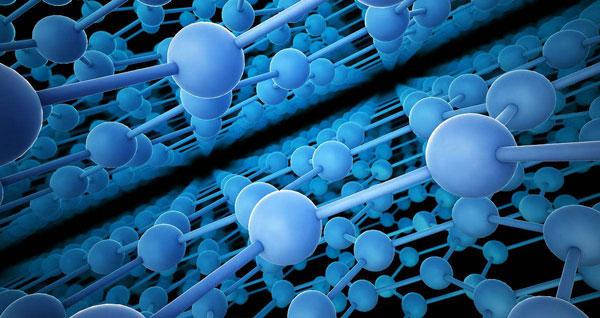
Graphene is a two-dimensional atomic crystal that consists of a single layer of carbon atoms arranged in a hexagonal lattice structure. It is known for its unique properties, including high energy density, strength, and conductivity. However, like all materials, graphene can be shaped and oriented in different ways, depending on how it is manufactured and processed.
(which substance has the most irregular shape caused by “unorganized” bonding?graphene)
One way to obtain irregular shapes from graphene is through the process of co-polymerization. This involves polymerizing a monomer of graphene (such as graphene nanotubes or graphene sheets) onto another material, such as a substrate. The resulting material will have the shape of the graphene monomer used to make it. For example, if one monomer is graphene nanotubes, the resulting material may be cylindrical or other irregular shapes.
Another method for producing irregular shapes from graphene is through chemical vapor deposition (CVD), which is a technique used to deposit thin layers of materials onto surfaces. In this method, carbon atoms are vaporized and deposited onto a surface, creating a film of graphene that can be shaped and oriented in various ways. For example, a CVD graphene film may have lines, circles, or other irregular patterns in it.
In addition to these manufacturing methods, graphene can also be shaped using mechanical means, such as cutting or engraving. These methods allow for more precise control over the shape and orientation of graphene, but they may not produce perfectly regular shapes.
(which substance has the most irregular shape caused by “unorganized” bonding?graphene)
Overall, while graphene is an excellent material with many potential applications, its irregular shapes can be challenging to obtain and manipulate using traditional manufacturing methods. However, with advances in technology, it may become possible to create graphene-based materials with highly controlled shapes and structures.
Inquiry us