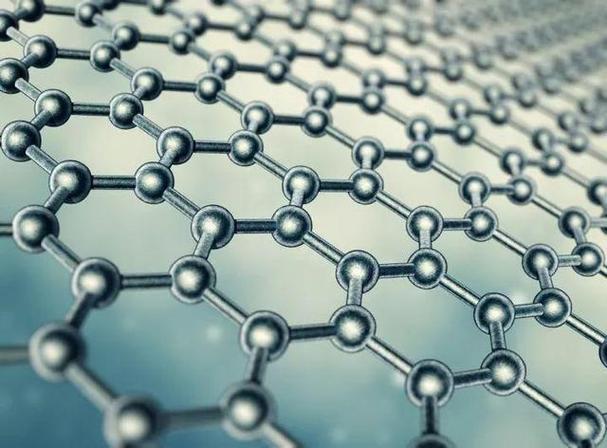
Graphene is a two-dimensional material made from a single layer of carbon atoms arranged in a hexagonal lattice. It has unique properties that make it an ideal material for various applications, including electronics, energy storage, and medicine.
(which type of substance is graphene?)
One of the most important properties of graphene is its electrical conductivity. Graphene is incredibly high in conductivity, making it well-suited for use as an electrode or in electronic devices. Its unique structure allows electrons to move through the material more easily than in traditional conductors, resulting in faster charge transfer.
Another advantage of graphene is its high temperature stability. Unlike traditional metals, which can be prone to corrosion at high temperatures, graphene can withstand temperatures of up to 400 degrees Celsius without losing its structural integrity. This makes it an ideal material for use in high-temperature applications, such as fuel cells and superconducting materials.
Graphene also exhibits exceptional mechanical strength and resilience. Despite its low density of just 1 gram per cubic meter, graphene is highly resistant to deformation and maintains its shape over long periods of time. This makes it an ideal material for use in structures that require high durability, such as bridges and buildings.
Graphene’s remarkable properties have led to numerous applications across various industries. In the electronics industry, graphene is being used as a replacement for traditional metals in electronic devices due to its high conductivity and thermal stability. Graphene-based batteries are also being developed, with potential for longer-lasting and safer batteries compared to traditional lithium-ion batteries.
In the energy storage industry, graphene has been explored as a viable alternative to traditional batteries due to its high energy density and fast charging capabilities. Graphene-based capacitors and energy storage systems have shown promise in terms of reducing costs and increasing efficiency.
Graphene is also being studied for its potential applications in medicine. Its exceptional mechanical strength and flexibility make it a promising material for use in biodegradable implants and artificial joints. Additionally, graphene’s high temperature stability and conductivity make it an ideal material for use in thermal energy storage systems.
Despite its many advantages, graphene faces several challenges when it comes to commercialization. One of the biggest challenges is the cost of producing graphene, which is currently prohibitively expensive. However, research efforts are underway to reduce the cost of graphene production by developing new synthesis methods and optimizing existing processes.
Another challenge facing graphene is its limited availability. While there are currently large quantities of graphene available, much of it is found in the form of thin films or powders that need to be synthesized using special equipment. This limits the number of applications that can benefit from graphene, particularly in industries that rely on mass production.
(which type of substance is graphene?)
Overall, graphene has enormous potential as a revolutionary material that could transform various industries. However, it will require significant investment and research to fully realize its potential. As researchers continue to explore the properties of graphene and develop new applications, we can expect to see even more exciting developments in the years ahead.
Inquiry us